Researchers Identify Tool to Detect Total Joint Replacement Surgeries that are Starting to Fail
New York—April 27, 2011
A recent study has demonstrated that doctors may soon have a tool for identifying orthopedic prostheses that are becoming loose after total joint replacement surgery, the most common reason joint replacements fail. The study shows that a minute molecule designed with novel properties can be used to identify patients who are at risk for failure and potentially deliver drugs to stop this process.
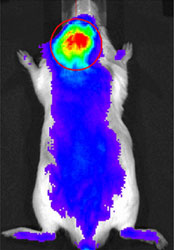 |
| A nanocarrier tagged with a near infrared fluorescent marker gravitated toward inflammatory cells that had been injected into the area over the scalp. |
“You can use this technique to visualize inflammation around the implant and that can alert the surgeon that the patient is at risk for loosening or we could use the system to deliver a drug that would block the inflammation that is responsible for the loosening process,” said Steven Goldring, M.D., Chief Scientific Officer, St. Giles Chair, Hospital for Special Surgery (HSS), who was involved with the study. The study, a joint collaboration between researchers at HSS and the University of Nebraska Medical Center, is published online, ahead of print, in the journal Molecular Pharmaceutics
Every year, roughly 1.5 million total joint replacement surgeries, such as total hip or knee replacement procedures, are performed worldwide. The overall 10-year success rate for these surgeries is 90 percent with close to 10 percent of patients requiring revision surgery, which is not always successful. Up to 20 percent of patients who undergo revision surgery for total knee replacements, for example, may still experience pain following surgery for months or even years.
The most common reason for a failed joint replacement surgery is the prosthesis becoming loose over time. “The loosening can be initiated by breakdown of the prosthetic materials that generates ‘wear debris’ composed of particles of the prostheses that are released into the tissues surrounding the prosthetic implants,” explained Dr. Goldring. “Those particles are very inflammatory, and they accumulate between the bone and the prosthesis. The local inflammation destroys the bone and the prosthesis becomes loose.” This is known as osteolysis.
“Osteolysis is the most common, long term complication of joint replacement surgery and it causes the need for revision surgery, which is associated with increased complications and is an expensive procedure,” said Ed Purdue, Ph.D., associate scientist and director of the Osteolysis Laboratory at HSS. “There are no real medical therapies for osteolysis beyond having a revision surgery, and it is a very difficult disease to track.”
While imaging modalities such as X-rays, computed tomography scans, and magnetic resonance imaging scans can identify bone damage and implant loosening, doctors would ideally like to have a tool that identifies the early roots of the problem, when the inflammation begins, not after the bone is damaged. “When pain or clear radiographic evidence is reported, unfortunately, considerable bone loss has already occurred, which cannot be easily restored,” said Dong Wang, Ph.D., associate professor in the Department of Pharmaceutical Sciences, University of Nebraska Medical Center.
If doctors can diagnose the problem early, they may be able to suppress the inflammation with drugs or perhaps replace the lining of a prosthesis, a very involved procedure.
Several years ago, Dr. Wang developed a nanomedicine containing a potent anti-inflammatory drug. The nanocarrier he used in this development had been used in the past to deliver chemotherapeutic agents to cancer cells. The system targets cancer cells by honing in on the abnormal blood vessel growth that is involved in carcinogenesis. Investigators at HSS and Nebraska Center wondered whether this system could be used to identify abnormal blood vessel growth and inflammation that is involved in other conditions and possibly deliver treatments. In 2006, they showed that it could potentially be used to target inflammatory arthritis joints. In the new study, they tested the tool’s ability to identify prosthesis loosening.
Performing an entire joint replacement surgery would be complicated in a mouse, so the researchers took a shortcut to mimic what happens in a human joint replacement that fails. The researchers injected wear particles from a human joint replacement into mice; specifically, they injected the particles into the area over the scalp. This generated an inflammatory reaction similar to what would be seen in humans in a joint replacement that was deteriorating. They then injected the nanocarrier tagged with a near infrared fluorescent marker into the bloodstream and showed that these nanocarriers gravitated toward the inflammatory cells. Autopsies of the mice verified this finding. Investigators then showed that the nanocarrier system could deliver dexamethasone, an anti-inflammatory drug, directly to the site of inflammation where it effectively inhibited inflammation and prevented osteolysis.
“One of the problems with the systemic delivery of drugs is that they act throughout the body on normal tissues leading to serious side effects,” Dr. Goldring said. “With this nanoparticle system, you avoid systemic toxicity and deliver the drug selectively to the site of inflammation.”
Dr. Purdue pointed out that, most likely, doctors will ultimately use therapeutic agents other than dexamethasone to treat osteolysis but the paper provides a proof of principle.
The researchers say that the nanocarrier system will need to be tested in an animal model with a prosthetic joint and then in a human, but they believe that they can rapidly move from this proof of principle study into human studies. “One of the advantages of this is that the nanocarrier system has already been approved in Europe and the United States for human phase I or phase II trials to improve anticancer chemotherapies, so we have already overcome one of the regulatory barriers,” Dr. Goldring said. “What we are now looking into is finding other therapeutic agents that we can couple to the nanocarriers, to more specifically target the inflammatory process induced by the orthopedic wear particles.”
Investigators at HSS and the University of Nebraska have filed a joint patent application on using the nanocarrier system for detecting prosthesis loosening.
The study was funded by the National Institute of Arthritis, Musculoskeletal and Skin Diseases and the American College of Rheumatology Research and Education Foundation.
Other Hospital for Special Surgery authors involved with the study are P. Edward Purdue, Ph.D., and Lyndsey Burton. University of Nebraska Medical Center researchers involved include Ke Ren, Ling-dong Quan, Edward Fehringer, M.D., and Geoffrey Thiele, Ph.D.
Need Help Finding a Physician?
+1.877.606.1555
Media Contacts
212.606.1197
mediarelations@hss.edu