An In-Depth Topic Review of Gout
For Physicians
- Definition
- Pathogenesis
- Clinical Presentation
- Laboratory Findings
- Differential Diagnosis
- Initial Treatment
- Long-term Management Issues
- Prognosis
- Future Possible Alternatives for Gout Management
- When to Seek Referral to a Specialist
- Annotated References
I. Definition
Gout is the arthritic syndrome due to the deposition of monosodium urate crystals. The attacks tend to come in discrete episodes, with normal joints in the intervening period, until late stages of the disease. Gout attacks often are monoarticular, but polyarticular episodes can occur.
Primary gout is not associated with an identifiable cause, other than perhaps a family history. Secondary gout refers to the presence of a recognized cause or precipitating factor, such as lymphoma (especially following chemotherapy), the excessive use of alcohol, or the use of diuretics (see Pathogenesis below).
II. Pathogenesis
Gout develops as a result of the build-up of purines in the body, either by decreased excretion (about 90% of cases of primary gout) or by increased production (about 10% of primary gout). When the concentration of urate exceeds its solubility, crystals precipitate, and the crystals are phlogistic. The crystals lead to activation of the classical and alternative pathways of complement, the influx of neutrophils into the joint, and the release of numerous inflammatory cytokines. In those patients who are over-producers of uric acid, their 24-hour urinary uric acid will likely be elevated, and they will be at risk of urate kidney stones as well as gouty attacks.
Gout is associated with atherosclerosis, hypertension and renal insufficiency, but there remains debate as to whether the association is independent. That is, debate exists as to whether patients with renal disease, for example, have elevated serum uric acid levels due purely to their decreased glomerular filtration rate or whether the hyperuricemia itself can damage the kidney (and whether uric acid is a risk factor for hypertension and coronary artery disease). In rats, data suggest that hyperuricemia has a direct effect on renal blood vessels.
Until the age of menopause, women lag far behind men in the incidence of gout. After menopause, the numbers become closer to equal, but still favor men.
Any factors that raise nucleoprotein production will increase its breakdown -- ultimately, by the action of xanthine oxidase -- into uric acid. Factors that decrease the secretion, or increase the reabsorption, of uric acid will likewise increase the risk of gout.
Secondary factors that may contribute to the elevation of serum urate and the development of gout include:
- chronic kidney disease, which impairs urate excretion;
- lead poisoning, which similarly reduces urate excretion;
- use of medications that reduce urate excretion, including diuretics, low-dose aspirin and cyclosporine;
- significant alcohol intake, which both increases urate production and decreases excretion;
- psoriasis, related to increased skin cell turnover and resultant hyperuricemia from nucleoprotein breakdown;
- lymphoma, especially during chemotherapy, due to cell turnover;
- the post-operative state and other physical stresses, such as myocardial infarction and cerebrovascular accident.
III. Clinical Presentation
From a diagnostic and therapeutic point of view, gout is most efficiently seen in three distinct stages: acute gout, inter-critical (between attacks) gout, and chronic tophaceous gout.
A. Acute gout
In about 70% of acute gout attacks, a single joint becomes suddenly inflamed, often with excruciating pain, erythema, swelling and exquisite tenderness. The attack may be accompanied by chills and a low fever. If untreated, the attack generally peaks within 24 hours and may linger for weeks, especially if the patient continues to use the affected joint actively. Those who get one attack of gout will generally get further attacks, usually within 2 years; 62% have a second attack within a year - and 90% within 10 years. However, 10% never experience an attack again.
The first metatarso-phalangeal (MTP) joint is the single most likely joint to be affected by gout, but many other joints can be involved. The mid-foot, ankle and knee are, respectively, the next most common locations, and the olecranon bursa may also be involved. Gout attacks in Heberden's nodes, especially in elderly women, have been frequently described. Gout is sufficiently rare enough in the shoulders and hips as to suggest that a different cause may be present, even in patients with known gouty arthritis.
Other diseases can also affect the first metatarso-phalangeal joint, such as pseudogout and Reiter's syndrome, (see Differential Diagnosis below), so involvement of this joint does not provide a definitive diagnosis of gout.
B. Inter-critical gout
Patients seen between gouty attacks generally appear normal - with no symptoms and with unremarkable joint examinations. The exception to this is in patients with long-standing gout who develop chronic tophaceous gout. The greatest controversies in the treatment of gout concern patients in the inter-critical phase.
C. Chronic Tophaceous Gout
After multiple gouty attacks, some permanent changes may occur in the joint, including joint damage and large collections of uric acid, or tophi.
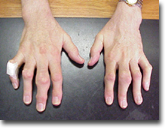
Multiple tophi in the hands of a patient with long-standing gout.
Tophi can drain the white uric acid crystals to the surface, which presents an infection risk.
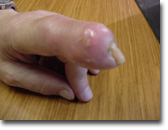
Gout tophi draining to the surface of the skin over a distal inter-phalangeal joint of the finger.
Patients with tophi are often over-producers of uric acid and therefore have elevated 24-hour urinary uric acid and are at risk of kidney stones.
IV. Laboratory Findings
The great majority of patients with gout have hyperuricemia. However, this is not always true, and some patients drop their serum urate levels in the setting of an acute attack.
Since gout is a life-long disease, often requiring life-long therapy, the importance of a definitive diagnosis cannot be over-emphasized. When a patient first presents with possible gout, it is almost always optimal to drain synovial fluid to examine for crystals. The needle-shaped urate crystals, seen as strongly negatively birefringent under polarized light microscopy, confirm the diagnosis. In some cases, the definitive diagnosis is even easier, such as when the patient has a tophus on the ear, where a scrape of the skin will provide material for crystal analysis, or when a tophus is about to break through to the skin - and a 27- or 30-gauge needle can be used to puncture the skin and obtain material for crystal analysis. It has been well-documented that urate crystals can even be found in asymptomatic metatarso-phalangeal joints. It is more difficult to aspirate a joint with minimal fluid, but often the diagnosis of gout can be made from only the tiny drop of serosanguinous fluid at the tip of an aspirating needle.
The 24-hour urinary urate determination can help when the prescriber is unsure whether to consider using probenecid as a uricosuric agent in a particular patient. When a patient is identified as a high urate excretor (i.e., an over-producer rather than an under-excretor), this is a significant push toward using avoiding the use of probenecid. Patients who excrete more than 800 mg of urate per 24h are considered over-excretors. When the decision is made to treat a patient with a xanthine oxidase inhibitor, such as allopurinol or febuxostat, then 24 hour uric acid determination may not be necessary, since decreasing the production of uric acid is effective therapy for both the overproducer and the underexcretor.
X-rays are often normal early in gout, but in later stages (e.g. chronic tophaceous gout) the x-rays can help distinguish gout from other conditions.
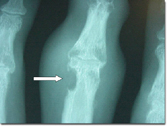
X-ray changes in chronic tophaceous gout. White arrow = erosion with overhanging edge.
X-rays in gout differ from those in rheumatoid arthritis in that there is no periarticular osteopenia. Erosions are seen in gout, but without surrounding decreased bone density.
V. Differential Diagnosis
Without crystal identification, great caution needs to be taken before making a diagnosis of gout, in view of the chronic treatment implications. The two "major criteria" for the diagnosis of gout are the documentation of urate crystals in either a joint or in a tophus.
Since certain clinical situations preclude crystal identification (e.g. patient refusal of arthrocentesis), "minor criteria" for gout diagnosis have been set up. To make the presumptive diagnosis of gout, six of the following 12 criteria should be present (note the importance of the history of a single joint with rapid and marked inflammation):
- more than one discrete arthritis attack;
- maximal inflammation within 24 hours;
- an episode of monoarticular arthritis;
- joint erythema;
- swollen or painful first MTP joint;
- unilateral inflammation of an MTP joint
- unilateral inflammation of a tarsal joint
- possible tophus;
- hyperuricemia;
- asymmetric joint swelling in a joint on X-ray;
- X-ray showing joint cysts and/or erosions without periarticular osteopenia;
- Joint inflammation with negative culture.
In addition, it's important to differentiate gout from pseudogout. Ideally, the differentiation is made by identifying the calcium pyrophosphate crystals under polarizing microscopy. When this is not possible, some clues to strongly consider pseudogout are:
1. calcifications seen in typical locations for pseudogout, e.g. the knee menisci, the triangular cartilage of the wrist, or surrounding the humeral head;
2. acute arthritis that seems like gout but is in a joint not usually involved by gout, such as the wrists, shoulders or elbows. (Gout is common in the olecranon bursa but rare in the elbow joint itself; osteoarthritis of the knees is much more prominent in the patello-femoral joint than in the medial or lateral compartment.)
VI. Initial Treatment
A number of different treatments are available for the management of acute gout. The options for managing chronic gout are more limited (see Long-Term Management Issues). A way to help patients think about the different approaches to gout treatment has been suggested by Dr. Robert L. Wortmann (see reference "1" below).
- Medications for Acute Gout
- Non-steroidal Anti-Inflammatory Drugs (NSAIDs) are the drugs of choice for acute gout. In the past, indomethacin was used due to limited alternatives. Some patients still find this drug to be the most effective, but it appears to cause more gastro-intestinal distress than most other such agents, and it can cause headache and dizziness, especially in the elderly. A variety of NSAIDs, such as naproxen and celecoxib (Celebrex®) are effective in gout, although recent years have seen more and more cases of acute gout managed with oral prednisone (see below) due to considerations of co-morbidities.
- Corticosteroids and ACTH, orally, intramuscularly or intravenously, are effective in gout. Studies many years ago suggested that there was a rebound effect in patients who took corticosteroids for gout, with flares on drug discontinuation, but recent studies have not shown this to be the case. A four- or five-day course of tapering doses of prednisone, e.g. 40 mg - 30 mg -20 mg -10 mg - will work in many patients. Some will need a longer course. Recent studies have shown prednisone to be at least as effective as NSAIDs for acute gout attacks, and likely a bit less toxic. Patients with ulcer disease or with a history of intolerance to NSAIDs, or those on warfarin, may be good candidates for corticosteroids. Patients with diabetes mellitus or infections are problematic with regard to prednisone therapy, but with close observation and management of the complicating disease, prednisone may still be possible. Local steroid injection should always be considered when practical for monoarticular episodes, in view of the relative safety of this approach (see below).
- Local injection of corticosteroids into a gouty joint is often an excellent alternative for acute monoarticular gout. The systemic risks are quite small, and the treatment is highly effective. In cases where the inflammation is more diffuse, as in the mid-foot, or when polyarticular involvement is present, this treatment may not be practical.
- Colchicine can be used orally to treat gout, and this treatment is often effective, although patients commonly develop diarrhea before attacks are resolved. Colchicine has recently been approved by the FDA for gout, although its use for this indication dates back to the time of Hippocrates. With the new FDA indication came a new dosage recommendation, from the study of Terkeltaub et al. The recommended colchicine dosage is 2 tabs (0.6mg each) followed by one tab (0.6mg) an hour later, with no further colchicine. This regimen had the same benefits at 24 hours as the previously used hourly colchicine regimen and had much less diarrhea. Drug interactions should be considered in prescribing colchicine, since serious interactions have been described with drugs such as clarithromycin (Biaxin®) and cyclosporine.Intravenous colchicine is almost never used, despite a high level of effectiveness, due to reports of bone marrow suppression and death with this treatment. Such deaths have been essentially confined to patients with renal insufficiency. The therapeutic window for intravenous colchicine is very narrow, and an error in dose, e.g., 10mg instead of 1mg, can be fatal. Oral colchicine is used with significant caution in patients on hemodialysis because colchicine cannot be hemodialyzed. On occasion, small doses, such as 0.6mg once or twice a week, can be used in that setting.
-
Other factors in management of acute gout
- Resting of the involved joint is important in managing acute gout. When a patient continues to walk on an inflamed metatarso-phalangeal joint, for example, this can prolong the attack.
- Timing of treatment is important. The speed of recovery of a gouty joint is often related to how long after the attack begins that the therapy is started. For example, if a patient begins treatment of a gout attack within an hour of its onset, the attack can often be resolved within a day. If the treatment starts after a week of gout, however, it may take a number of days for the attack to resolve.
VII. Long-term Management Issues
A. Diet
- Purine intake still plays a role in the management of gout. Although even a very strict low purine diet can only reduce the serum urate by ~1.0 mg, some patients notice a significant reduction in gout attacks when watching their diet carefully (See reference #2 below for diet books that may be helpful to your patients.) The most important dietary advice includes telling patients to avoid foods with very high purine content such as organ meats (liver, kidney, hearts, sweetbreads [thymus and pancreas]), also known as offal, anchovies, salmon, sardines, meat extracts, and meat gravies.
- Red meat and shellfish should also be reduced in the diet.
- Recent data suggests that vegetables are not a major issue in causing the onset of a gout attack, and low-fat dairy products have been shown to have a degree of preventive effect in gout onset.
- Alcohol intake needs to be limited, since alcohol decreases the excretion of uric acid and also increases its production. Patients with gout should try to eliminate beer from their diet if at all possible.
- Fluid intake of at least 4-5 cups a day can help in preventing kidney stones.
B. Medications for Inter-Critical Gout
- General principles: Four alternatives are available prophylaxis during the period between attacks: allopurinol, colchicine, probenecid and sulfinpyrazone.
- Colchicine prophylaxis, used at 0.6 mg qd or bid, helps in preventing gout attacks, although the uric acid level is unchanged. Some patients will get diarrhea with 0.6 mg bid, and some of those will be able to tolerate the drug once a day. Colchicine prophylaxis for 6 months, one or two tabs daily, is generally given when uric acid-lowering therapy is begun. If a patient has tophi, this prophylaxis is often continued indefinitely.
- Uricosurics: Probenecid is used to prevent gout attacks in patients who are found not to be at risk of kidney stones. (This will require 24-hour urinary uric acid testing.)
Because of their relative safety, uricosuric drugs are good choices for patients with recurrent gouty attacks despite colchicine prophylaxis in those who have a creatinine clearance greater than 80 mL/m. (These medications are not effective in the setting of renal insufficiency). Uricosurics prevent reabsorption of uric acid by the kidney, and therefore their major risk is kidney stone. Thus, once a patient is placed on a uricosuric agent, it is reasonable to follow up on the 24-hour urinary uric acid to be sure that the amount has not risen above ~600 mg/24h. If it does, then the gout risk may be lowered while increasing the risk of kidney stone. Patients on uricosurics also need to be sure to take sufficient water to maintain a strong urine flow.
A new uricosuric agent, presently known only as RDEA 594, is currently under investigation. This agent can be used once a day, rather than the twice-daily probenecid, and has other features that might make it easier to use than probenecid. - Allopurinol (Zyloprim® and others) and febuxostat (Uloric®) are xanthine oxidase inhibitors, which decrease the body’s production of uric acid. Allopurinol has been available for over 40 years, and febuxostat was approved in February of 2009. In patients who are overproducers of uric acid (with >800mg of urinary uric acid in 24 hours) or with tophi, or those who are not appropriate candidates for uricosuric agents, allopurinol and febuxostat are drugs to consider. For many patients, allopurinol is the first line drug for lowering uric acid, and febuxostat can be used if allopurinol is not tolerated or ineffective. As noted below, febuxostat can also be considered first line in patients with mild to moderate renal dysfunction (e.g., estimated creatinine clearance 30-80 mL/min).
Allopurinol is indicated in patients about to receive chemotherapy for malignancy, to prevent gout due to cell breakdown, but febuxostat does not at this time have this indication. Febuoxstat does not have an indication for the treatment of patients without gout who have uric acid kidney stones, but allopurinol does have this indication. Febuxostat has the advantage of having only 3% renal excretion, while allopurinol (and its active metabolite oxypurinol) have ~ 90% renal excretion. For this reason, in patients with mild to moderate renal insufficiency (creatinine clearance 30-80mL/min), the dose of febuxostat (40mg to start, then 80mg if uric acid remains > 6.0) is unchanged.
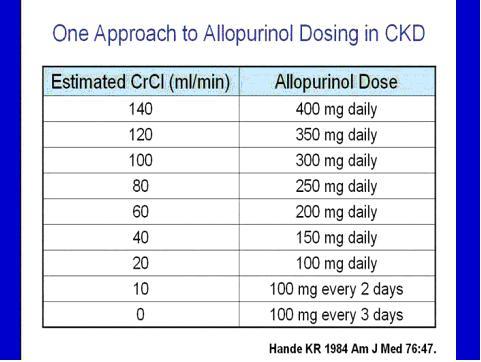
With allopurinol, dosage adjustment is required, with one published recommendation as follows:
Allopurinol has, rarely, caused a severe allergic reaction, the so-called “allopurinol hypersensitivity syndrome,” with leukopenia, fever, very severe skin rash, and a significant mortality. It remains unclear whether this is a dose-related phenomenon, although one uncontrolled case report described all 11 cases with this syndrome as having renal insufficiency, raising the question of allopurinol accumulation. Febuxostat may avoid this issue of accumulation in patients with mild to moderate renal insufficiency. Recent studies, so far presented only in abstract form, have reached different conclusions as to whether renal insufficiency is a risk factor for allopurinol hypersensitivity.
In the first two Phase 3 trials of febuxostat, a numerical but statistically insignificant increase in cardiovascular adverse outcomes was noted in the febuxostat arms as compared to allopurinol. A third, much larger phase 3 trial was required by the FDA, which had no numerical increase in such outcomes. Combining all phase 3 trials and long-term extension studies of febuxostat, there is a numerical, non-statistically significant, increase in adverse cardiovascular outcomes, which the FDA notes does not demonstrate febuxostat to be identified as causal. Other adverse effects were similar in the allopurinol vs febuxostat arms of the trials. In deciding between allopurinol and febuxostat, the long-term use of allopurinol, its lower cost, and the history of serious allopurinol hypersensitivity reactions need be seen as factors to consider re: allopurinol.
The low renal excretion of febuxostat, along with the demonstration that 80mg of febuxostat is more potent in getting patients to the goal of uric acid < 6, may indicate this agent in specific patients. Febuxostat is a more expensive agent than allopurinol, and has a much shorter overall clinical experience, but it is noted that there is more data available on more patients on febuxostat than has been published with allopurinol since allopurinol was released. It should also be mentioned that there is very little data available on the safety and efficacy of doses of allopurinol > 300mg, especially in patients with renal insufficiency, despite rheumatologists often using such doses out of necessity. Some patients may benefit from a switch to febuxostat as an alternative to very high doses of allopurinol.
An intravenous and an oral desensitization protocol are available for allopurinol, but some may wish to try febuxostat in a patient allergic to allopurinol. No formal studies have addressed the potential for cross-allergenicity of allopurinol and febuxostat, but the chemical structure of the two agents is quite different. Patients who begin on allopurinol or febuxostat will, not uncommonly, develop an increase in gouty attacks in the months following initiation of this medication. For this reason, it is generally recommended to start colchicine prophylaxis along with the xanthine oxidase inhibitor, and continue the colchicine for the first six months of allopurinol therapy. In patients with tophi, often the prophylaxis is continued indefinitely, since the cause of the flares after starting uric acid lowering appears to be uric acid mobilization, and tophi can take years to fully mobilize and be reabsorbed.
- Uricase: Pegloticase (Krystexxa®) has recently been approved for gout. This intravenous agent is a pegylated mammalian uricase which breaks down uric acid to the more soluble allantoin, which can be readily excreted. Pegloticase is our most potent uric acid lowering agent, and can take a uric acid of 12 down to 1 in one day, and appears to be the most effective agent available to shrink tophi quickly. This agent has a major role for patients who have not been able to get their uric acid below 6 despite xanthine oxidase inhibitor therapy. Caution is needed with pegloticase because of infusion reactions that have been described, including anaphylaxis. It appears that a number of these infusion reactions can be avoided by stopping this agent in any patient whose uric acid before the infusion is >6 on two consecutive testings. Failure to lower uric acid after a pegloticase infusion is highly related to the development of high levels of antibodies to PEG These antibodies appear to both decrease effectiveness of the agent and also increase the likelihood of infusion reactions.
General considerations in the management of chronic gout require taking each patient on an individual basis.
Considerations regarding long-term treatment of gout include:
- Compliance
- Variable severity
- Joint damage variable
- Concurrent illness variable
When choosing to use no prophylaxis, versus colchicine, a uricosuric or xanthine oxidase inhibitor, one must note whether the patient is likely to comply with treatment. Allopurinol, for example, carries some long-term risk of liver toxicity, and may not be ideal in a patient who cannot be counted on to have lab tests on a 6-12 month basis. The variability of gout severity and joint damage makes it important to assess these issues in each patients. Concurrent illnesses, such as alcoholism, renal insufficiency and colitis, would all have an impact on the choice of chronic medication for gout.
C. Management of Chronic Tophaceous Gout
- Medical therapy of tophi: Allopurinol or febuxostat are essentially always indicated in patients with chronic tophaceous gout. In those not suitable for xanthine oxidase inhibition, pegloticase is an alternative option.
- Surgery to remove tophi that are beginning to break through to the skin is sometimes needed and can prevent severe infectious problems and osteomyelitis. It can take 18 months or more for allopurinol or febuxostat to lead to tophus resorption, and perhaps 3 months with pegloticase. Some patients have tophi which need to be addressed with a more urgent timetable due to active or impending infection.
D. Management of Patients with Gout and Kidney Stones
- The presence of kidney stones makes it necessary to approach uric acid lowering with a xanthine oxidase inhibitor rather than a uricosuric agent. Allopurinol is indicated by the FDA for prevention of kidney stones. Febuxostat is indicated for gouty patients with elevated uric acid levels, and despite a present lack of indication would be expected, like allopurinol, to lead to a decrease in urinary uric acid. Uricase, if it becomes available, would also be an option in patients with urate kidney stones, since this agent will also lower urinary uric acid.
- Fluid intake should remain high.
- Urinary alkalinization, as with potassium citrate combined with citric acid, can increase the solubility of urate and decrease the risk of urate crystallization.
E. Management of hypertension, obesity, hyperlipidemia, and coronary disease in patients with gout
Gout as a clue to the above conditions is important in that they should be sought in any patient with gout. It appears to be quite clear that hyperuricemia is an independent risk factor for coronary artery disease. It has not been proven as yet that lowering uric acid improves outcome in patients with coronary artery disease, so at this time it cannot be justified to treat asymptomatic hyperuricemia on the basis of cardiac prevention or treatment. It should be noted that patients with gout quite often have many cardiac risk factors, and they should be closely monitored and managed in terms of risk factor reduction.
VIII. Prognosis
Response to treatment is generally quite good, and most patients with gout can be managed with allopurinol (or probenecid) or febuxostat.
IX. Future Possible Alternatives for Gout Management
For treatment of acute gout, there is some early case report evidence that the IL-1 receptor antagonist anakinra is helpful for acute gout in patients where other therapies are ineffective or contra-indicated. Two longer-acting IL1 blockers are under study [canakinumab (Ilaris®) and rilonacept (Arcalyst®)], both for acute treatment of gout and for prophylaxis against gout attacks early in the course of uric acid lowering therapy.
X. When to Seek Referral to a Specialist
Most patients with gout can be managed by their primary care physicians. Cases where a rheumatologist may be helpful include:
- the involved joint is difficult to aspirate and crystal identification is needed;
- the diagnosis is unclear;
- the patient cannot tolerate the usual first and second line agents to lower their uric acid;
- the patient continues to have gout attacks despite a prophylactic regimen;
- the patient has both gout and significant renal insufficiency;
- large tophi have already or are threatening to break through the skin.
XI. Annotated References
A. Wortmann RL. Effective management of gout: an analogy. Am J Med. 1998 Dec;105(6):513-4. A review article that includes the "match" analogy to help patients understand the management of the various stages of gout.
B. Schneiter J. Gout Hater's Cookbook: Recipes Lower in Purines and Lower in Fat. (Reachment Publications; 2000) In addition to comprehensive lists of foods lower, relatively high, and highest in purines, this book offers nearly 100 low-purine recipes.
C. Schneiter J. Gout Hater's Cookbook II: The Low Purine Diet Cookbook. (Reachment Publications: 2001) More recipes from the same author. This book is useful for your patients, who often find the recommendations about low purine diets confusing and difficult to follow even when well understood.
D. Wallace SL, Singer JZ. Review: Systemic toxicity associated with the intravenous administration of colchicine - Guidelines for use. J Rheumatol 1987;15:495-9. This is an important article on potentially lethal complications of intravenous colchicine; it emphasizes the importance of renal insufficiency as a risk factor for complications.
E. Singer JZ, Wallace SL. The allopurinol hypersensitivity syndrome: Unnecessary morbidity and mortality. Arthritis Rheum 1986;29:82-6. This article stresses the importance of renal insufficiency as a risk factor in allopurinol hypersensitivity, and the importance of reducing allopurinol dose in patients with renal insufficiency and of making sure that only patients who meet appropriate criteria get treated with allopurinol.
F. Moriwaki Y, Yamamoto T, Takahashi S, et al. Spot urine uric acid to creatinine ratio used in the estimation of uric acid excretion in primary gout. J Rheum 2001;28:1306-10. This article is part of an ongoing discussion among rheumatologists as to whether spot urinary urate/creatinine ratio can replace 24-hour urinary urate determination.
G. Fam AG, Dunne SM, Iazzetta J, et al. Efficacy and safety of desensitization to allopurinol following cutaneous reactions. Arthritis Rheum 2001;44:231. This article reviews a regimen of oral desensitization to allopurinol, which has had significant success with low risk.
H. Sundy JS et al: Reduction of Plasma Urate Levels Following Treatment With Multiple Doses of Pegloticase (Polyethylene Glycol–Conjugated Uricase) in Patients With Treatment-Failure Gout: Results of a Phase II Randomized Study. Arthritis Rheum 58:9, 2882-2891, 2008. This article showed the effectiveness of pegloticase in rapidly lowering uric acid level.
I. Baraf, HSB et al: Reduction of tophus size with pegloticase (PGL) in treatment failure gout (TFG): Results from Gout-1 and Gout-2, European League Against Rheumatism Abstract OP-0047, June 2009. Abstract from European League Against Rheumatism Meeting 2009. Tophi were shown to be rapidly decreased in size.
K. So A et al: A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Research & Therapy 9(2):R28, 2007. IL-1 receptor blocker was shown effective in acute gout.
L. Terkeltaub R et al: The interleukin 1 inhibitor rilonacept in treatment of chronic gouty arthritis: results of a placebo-controlled, monosequence crossover, non-randomised, single-blind pilot study. Annals of Rheumatic Disease 68:1613-1617, 2009. IL-1 receptor inhibition was shown to be effective in acute gout, with a longer-acting blocker.
M. So A at al: Canakinumab (ACZ885) Vs. Triamcinolone Acetonide for Treatment of Acute Flares and Prevention of Recurrent Flares in Gouty Arthritis Patients Refractory to or Contraindicated to NSAIDs and/or Colchicine. American College of Rheumatology Abstract LB4, October 2009. Abstract from American College of Rheumatology Meeting, Oct. 2009. An extended-release blocker of IL-1 was effective in both treating flares and preventing the flares induced by urate lowering agents.
N. Yeh N et al: RDEA594, a potential uric acid lowering agent through inhibition of uric acid reuptake, shows better pharmocokinetics than its prodrug, RDEA806, poster #28, American College of Rheumatology Meeting, October 2008. Abstract from American College of Rheumatology Meeting 2008. A uricosuric is under study which may be easier to use and more effective than probenecid.
O. Fitz-patrick D et al: Abstract 150: Effects of a Purine Nucleoside Phosphorylase Inhibitor, BCX4208, on the Serum Uric Acid Concentrations in Patients with Gout. Abstract from the American College of Rheumatology Meeting November 2010. Another blocker of uric acid production is under study.
Authors
Attending Physician, Hospital for Special Surgery
Professor of Clinical Medicine, Weill Cornell Medical College