Recurrent Amaurosis Fugax in a Pregnant Patient with “Triple Positive” Antiphospholipid Antibodies and Prior Late Pregnancy Loss
From Grand Rounds from HSS: Management of Complex Cases | Volume 11, Issue 3
Case Report
A 32-year-old woman presented to HSS for evaluation after her first pregnancy ended in late miscarriage. The patient was known to have “triple” antiphospholipid (aPL) antibody positivity—lupus anticoagulant (LAC) and high-titer anticardiolipin and anti-β2-glycoprotein I immunoglobulin G—discovered on evaluation for steroid-responsive thrombocytopenia. Medical history also included ocular migraine, Raynaud’s phenomenon, and livedo reticularis. Family history was notable for antiphospholipid syndrome (APS) in 2 distant relatives. Antinuclear antibody and other lupus-related autoantibodies were absent; serum complement levels (C3 and C4) were low.
At 14 weeks’ gestation, she began experiencing weekly episodes of amaurosis fugax (AF) affecting the right eye—“like a gray shade descending”—lasting seconds to minutes. At 17 weeks, she had a spontaneous abortion requiring dilation and evacuation (D&E); fetal anatomy and karyotype were normal. She received prophylactic low molecular weight heparin (LMWH) 30 mg daily for 6 weeks. By 3 weeks after the D&E, she had AF episodes once every 3 days and reported a 10-minute episode of confusion. Magnetic resonance imaging (MRI) of the brain identified a left cerebellar lesion (Fig. 1A). She was started on low-dose aspirin (LDA; 81 mg daily) and symptoms resolved; an MRI 4 weeks later showed resolution of the cerebellar lesion (Fig. 1B).
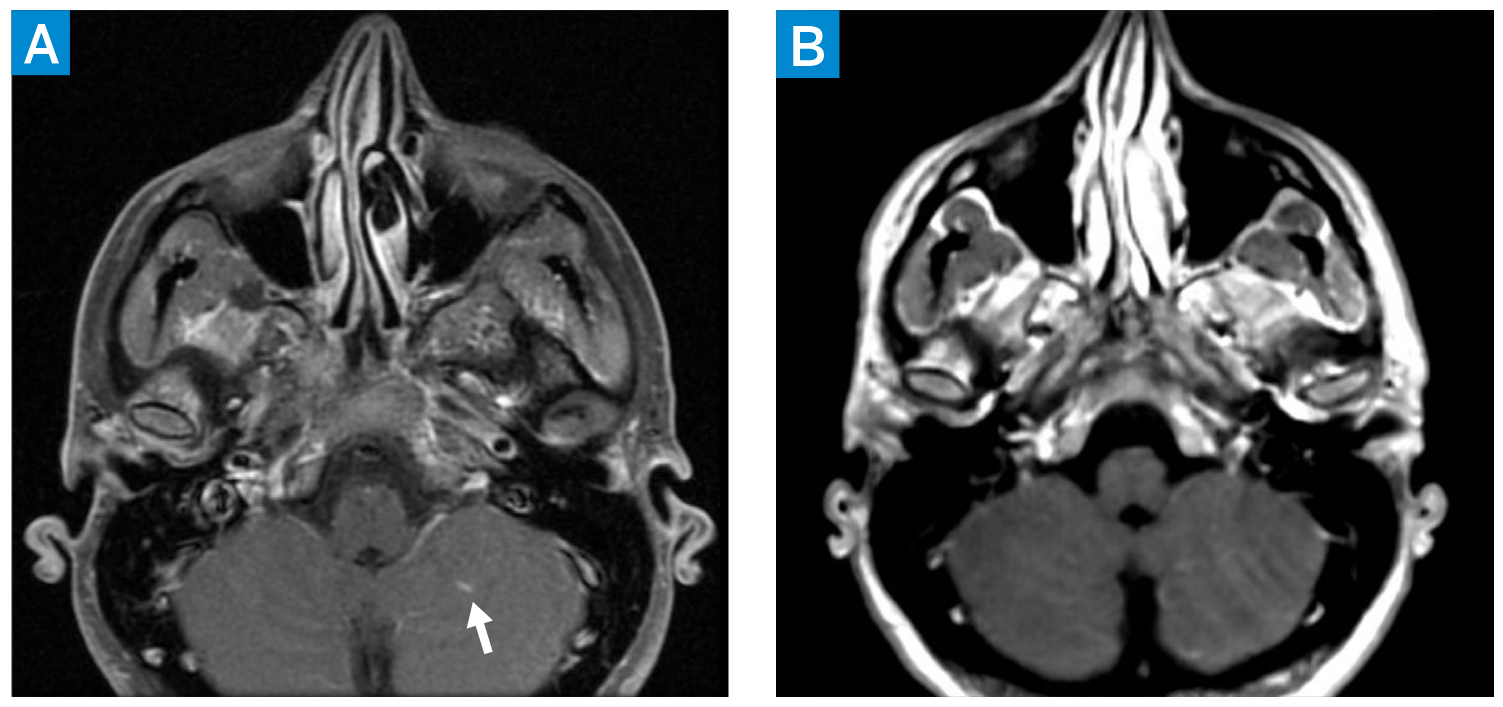
Figure 1: Axial postcontrast T1 MRI of the brain (A) demonstrating left cerebellar lesion with associated enhancement (arrow). Initial differential included subacute ischemia and a benign low-flow vascular malformation. Axial postcontrast T1 MRI of the brain 4 weeks later (B) showing resolution of enhancement. Taken together, the findings are consistent with subacute ischemia on the first MRI (A), evolving into chronic ischemia on the second MRI (B).
She conceived 4 months after the D&E, while taking prophylactic LMWH and LDA. Given the history of recurrent AF and the cerebellar lesion, her team (rheumatology, maternal–fetal medicine, hematology, and neurology) increased LMWH to therapeutic dosing at 60 mg twice daily. Also, from 8 to 28 weeks’ gestation, she received certolizumab via a clinical trial [1]. This pregnancy was uneventful until 25 weeks’ gestation, when she had an episode of AF. MRI of the brain and neck was normal. The anti-factor Xa level was subtherapeutic, and LMWH was increased to 70 mg twice daily. Hydroxychloroquine (HCQ) 300 mg and magnesium 500 mg daily were added. At 31 weeks, she again experienced AF; the anti-factor Xa level was at the low end of the therapeutic range, and LMWH was increased to 80 mg twice daily. At 37 weeks, she had a planned induction of labor and an uncomplicated vaginal delivery and was discharged home on therapeutic LMWH, LDA, HCQ, and magnesium.
Discussion
Patients with APS and persistent LAC, or “triple positive” aPL, experience a high rate of adverse pregnancy outcomes, despite treatment with LDA and prophylactic LMWH [2, 3]. Our patient was treated with LDA and, once there was high suspicion for transient ischemic attack/stroke, with therapeutic LMWH, in accordance with the 2020 American College of Rheumatology guideline for management of thrombotic APS in pregnancy [4]. In addition, she received certolizumab as part of an ongoing, open-label clinical trial [1]. In APS mouse models, tumor necrosis factor-alpha (TNF-α) has been identified as a mediator of fetal damage and pregnancy loss, and it is hypothesized that TNF-α inhibitors may decrease the risk of adverse pregnancy outcomes in patients with persistent LAC [1, 5]. Unlike other TNF-α inhibitors, certolizumab has minimal placental transfer [4]. Just before discontinuation of certolizumab, the patient started HCQ, which observational data suggest may decrease the risk for severe pregnancy complications in primary APS [4, 6].
Using medications that target both thrombotic and inflammatory mechanisms, this high-risk patient delivered a healthy, full-term infant. Our case highlights the importance of close monitoring and multidisciplinary collaboration to guide treatment before, during, and after pregnancy in patients with APS.
Authors
Attending Physician, Hospital for Special Surgery
Assistant Professor of Medicine, Weill Cornell Medical College
Attending Physician, Hospital for Special Surgery
Professor of Clinical Medicine, Weill Cornell Medicine
References
- IMPACT Study: IMProve pregnancy in APS with Certolizumab Therapy. ClinicalTrials.gov identifier: NCT03152058.
- Lockshin MD, Kim M, Laskin CA, et al. Prediction of adverse pregnancy outcome by the presence of lupus anticoagulant, but not anticardiolipin antibody, in patients with antiphospholipid antibodies. Arthritis Rheumatol. 2012;64(7):2311– 2318. doi: 10.1002/art.34402
- Ciccone G, Berghella V, Marotta GM, et al. Antiphospholipid antibody profile based obstetric outcomes of primary antiphospholipid syndrome: the PREGNANTS study. Am J Obstetric Gynecol. 2017;216(5):525.e1–525.e12. doi: 10.1016/j. ajog.2017.01.026
- Sammaritano LR, Bermas BL, Chakravarty EE, et al. 2020 American College of Rheumatology guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol. 2020;72(4):529–556. doi: 10.1002/ art.41191
- Berman J, Girardi G, Salmon JE. TNF-alpha is a critical effector and a target for therapy in antiphospholipid antibody-induced pregnancy loss. J Immunol. 2005;174(1):485–490. doi: 10.4049/ jimmunol.174.1.485
- Ruffatti A, Tonello M, Hoxha A, et al. Effect of additional treatments combined with conventional therapies in pregnant patients with high-risk antiphospholipid syndrome: a multicentre study. Thromb Haemost. 2018;118(4):639646. doi: 10.1055/s-0038-1632388


