Early Diagnosis and Treatment of Rheumatoid Arthritis
An overview for primary care physicians, physical therapists, and occupational therapists

Rheumatoid arthritis (RA) is the most common type of autoimmune inflammatory arthritis (IA), and the most common type of IA in general, other than gout (which is about six times more common). If detected and treated in its early stages, the effects of RA can be greatly diminished, and the condition may even remit completely.
The importance of proper diagnosis, particularly in the early stages of the disease, is often underestimated by primary care physicians, but it may prevent serious, lifelong arthritic complications for their patients. Many RA patients do not seek medical help because they erroneously assume nothing can be done for their arthritis. Others may experience delayed therapy because their primary care physicians are not familiar with current treatment advances. RA often begins with inflammation in a few joints. Once that pain persists for three or more months it is unlikely to go away on its own and often spreads to multiple other joints.
There is a therapeutic window of opportunity to prevent joint damage in RA, and that window opens early. Treatments are available to put the brakes on the progression of the disease. But the real key is avoiding delay in treatment. Waiting too long to be evaluated and treated by a rheumatologist can result in unnecessary joint destruction.
How do you differentiate between osteoarthritis and rheumatoid arthritis?
Rheumatoid arthritis (RA) is a form of arthritis in which joint inflammation is caused by an overactive immune system that affects the joint lining (also called the synovium). This can affect adults of any age, but most commonly starts in middle age. RA usually affects many joints throughout the body at the same time but can involve just one joint.
Is joint pain or swelling a new problem for you?
Call us directly for a fast track appointment
While the joint symptoms (pain, swelling and stiffness) are typically most severe, RA affects the whole body and can lead to generalized symptoms such as fever, weight loss, malaise, and fatigue. Some patients also have non-joint (extra-articular) manifestations such as nodules, eye inflammation or pulmonary fibrosis.
In contrast, osteoarthritis (OA) is primarily due to breakdown of cartilage and the issue is localized to the joint (there are no generalized symptoms). OA most typically presents after the age of 50 and becomes more common with age.
When symptoms present in the hands or feet, a simple test of the metacarpophalangeal (MCP) or metatarsophalangeal (MTP) joints can be performed, which, if positive, can suggest rheumatoid arthritis. In this test, the second through fourth metacarpophalangeal joints (see image, lower left) or the metatarsophalangeal joints (see image, lower right) are squeezed together to test for tenderness. A positive result raises the question of an inflammatory arthritis such as rheumatoid arthritis.
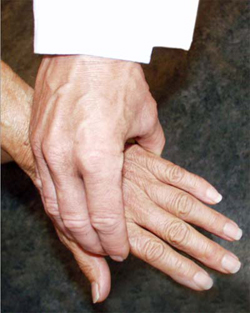
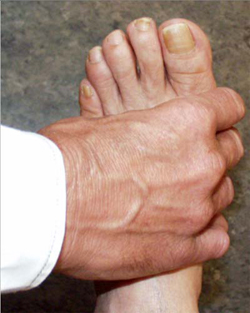
"Squeeze Test" of the MCP joints in the hand and MTP joints of the foot
What are the early signs of rheumatoid arthritis?
Multiple tests can be administered to detect the early warning signs of RA. The proper diagnosis of RA can include the following:
- Symmetrical inflammatory arthritis (affecting both halves of the body in the same joint groups), especially in the small joints of the hands, associated with morning stiffness in the joints lasting over one half hour.
- Morning stiffness in the joints lasting at least 30 minutes and often more than 60 minutes.
- Lab test results:
- Positive rheumatoid factor (less specific).
- Anti-CCP antibody elevation (more specific).
- Sedimentation rate or C-reactive protein elevation.
- X-ray changes: The first change on X-ray is often joint space narrowing. However, bone erosions near the joint may develop early in the course of RA. In addition, newer imaging techniques such as MRI and ultrasound detect erosions significantly earlier in the course of RA than were found in prior studies using X-rays.
Who gets rheumatoid arthritis?
RA is the most common form of autoimmune inflammatory arthritis. As previously mentioned, it affects as high as 1% of the world's population, occurs three times more often in women than in men, and in most cases, develops in adults between the ages of 25 and 50. However, children and the elderly are also at risk.
What are the benefits of discovering rheumatoid arthritis early?
RA requires early diagnosis; it can remit if left unchecked and undiagnosed in perhaps 5% of patients but will much more likely evolve into a chronic rheumatoid arthritis. Conversely, early treatment can prevent future deformity and disability, and will likely help reduce collateral damage, such as atherosclerosis.
In early arthritis there is a "window of opportunity”, during which the disease can be treated before irreversible joint damage occurs. This window varies between patients but is usually open 3 to 6 months after the onset of the disease. For this reason, RA should be addressed in a timely manner.
In a meta-analysis of 14 trials by the American College of Rheumatology (ACR), patients suffering from a shorter duration of their disease were more likely to show improvement than long-term sufferers when properly diagnosed and treated. This finding supports the concept of a window of opportunity, in that early treatment improves outcomes.
What are the complications of untreated rheumatoid arthritis?
When RA is left untreated, joint destruction is highly likely, leading to functional disability and high risk of:
- inability to work
- psychosocial dysfunction
- comorbid conditions, such as cardiovascular disease
- reduced life expectancy
- large economic loss, both to the individual and to society
What is the course of treatment for rheumatoid arthritis?
A multipronged approach is essential for RA treatment, including patient education, nonpharmacologic therapy, pain medications (such as NSAIDs), DMARDs and biologic agents, and/or surgery:
- Patient education: As early as possible after diagnosis, it is important for the patient to learn about his/her disease in order to anticipate its symptoms, effects, treatments, and potential outcomes. It is also useful to educate patients who have RA on strategies for lifestyle modifications and the use of assistive devices which may help them avoid exacerbating their symptoms and manage daily activities. Many of these also apply to patients with osteoarthritis.
- Nonpharmacologic therapy can be extremely helpful in patients' efforts to manage their disease – a physical therapist or occupational therapist is essential in aiding the patient's physical recuperation as concurrent pharmacologic treatment modalities are administered. The patient and therapist should focus on pain and spasm relief, strengthening and flexibility exercises, ambulation training, and assistive devices and splinting as indicated.
- NSAIDs (nonsteroidal anti-inflammatory drugs, such as ibuprofen or naproxen) can help with pain either before RA directed treatment is started by a rheumatologist or for break-through pain.
- Oral and intra-articular corticosteroids (such as prednisone or methylprednisolone), used judiciously, can work quickly and significantly improve the symptoms of RA. Due to side effects with long-term use of these medications, they are typically used for only short periods while starting RA-directed treatment or managing a flare up.
- DMARDs (disease-modifying antirheumatic drugs) are usually the first medications a patient takes for their RA. They are pills that help control the abnormal inflammation in the joint.
- Biologic agents are a group of newer treatments that target specific proteins and immune cells that contribute to inflammation in RA. They are typically given in the form of either an infusion (by a nurse) or an injection under the skin that is done at home.
- Surgery: A variety of surgical procedures may be needed in patients with RA. If the arthritic process has been uncontrolled for a long period, sufficient joint damage may occur in a joint such as the hip or knee that a total hip replacement or knee replacement may be needed. Other procedures used at times in patients with RA include a "cleanout" or synovectomy of an inflamed joint, such as the knee, which can be done with an arthroscopic procedure. Surgical repair of tendon ruptures by the inflammatory process, such as in the hand, can restore function.
What is the role of the primary care physician and physical therapist/occupational therapist in early rheumatoid arthritis?
Primary care physicians and physical and occupational therapists should be attuned to the early warning signs of RA and recommend early consultation with a rheumatologist for work-up and possible early intervention. They can also educate the patient about the systemic and destructive nature of rheumatoid joint disease and the "window of opportunity" presented by proper diagnosis of early arthritis.
Patients with early rheumatoid arthritis need a "team," and this includes the primary care physician, the rheumatologist, and the physical and/or occupational therapist. While the patient gets started on medical therapy, education about living with arthritis is important. All such patients need to be taught an exercise and stretching program, and many need splinting as well as muscle-relaxing and motion-enhancing therapeutic modalities (for example, ultrasound, heat, etc.). See a list of articles on these topics further below.
Conclusion
After gout, rheumatoid arthritis is the second most common type of inflammatory arthritis, and it is the most common form of autoimmune IA. It is a painful, disabling disease which shortens lifespan and can have a major impact on the systemic health and lifestyle of the patient. Early diagnosis and treatment of RA can markedly decrease disability and pain and very likely lengthen life, and the "window of opportunity" presented in the early stages of the disease must be recognized. Once the diagnosis of RA is made, disease modifying agents should be administered to essentially all RA patients.
RA is a condition that can be deforming, disabling and potentially life-shortening, but it is treatable – especially in its early stages. Treatment can still be quite effective even in the later stages of RA. Evidence has shown that patients with rheumatoid arthritis who are effectively treated can often avoid deformity and move their life span to the normal range. Stopping inflammation has been shown to not only stop joint swelling and pain, but also to decrease risk of heart complications.
Primary care physicians and physical/occupational therapists can make a vital difference in their patients' lives if they know how to detect the early warning signs of arthritis and take the proper measures to make sure the patient has a health care team which can provide all the elements to stop the arthritis in its tracks.
Updated: 3/3/2025
Authors
Attending Physician, Hospital for Special Surgery
Professor of Clinical Medicine, Weill Cornell Medical College


